The introduction of a safe and effective product by 2021 seems highly possible as multiple candidates have entered Phase II trials. Unprecedented research efforts, in terms of both speed and scale, by hundreds of research groups are anticipated to bring a fundamental and necessary change in the traditional pathway for vaccine development and commercialization. COVID-19 vaccine development did not seem to be an encouraging prospect as historically, the general time duration required to develop a vaccine has been about 20 years. For instance, the development of the Human Papilloma Virus (HPV) vaccine took around 26 years, the rotavirus vaccine was developed in 25 years. It took more than 50 years of research to introduce a vaccine for the Respiratory Syncytial Virus (RSV).
Request Sample Copy of the Report @ https://www.grandviewresearch.com/industry-analysis/covid-19-vaccines-market
Several initiatives undertaken by the government
regulatory bodies and the World Health Organization (WHO) can be attributed to
the rapid research in vaccine development. The Who and the Coalition for
Epidemic Preparedness Innovations (CEPI) have led the COVAX Vaccine initiative
for accelerating the development and increasing equitable access to each
participating economy. The bodies are also jointly working toward enhancing
manufacturing capabilities and efficient management of the buyer-supplier
chain, well ahead of time to enable a fair distribution of over 2 billion doses
by the end of 2021. As of August 2020, 172 countries are involved in the
discussion for potential participation in the COVAX. The initiative also aims
at filling key funding gaps by providing a way to support the participation of
lower-income economies in the COVAX Facility.
In addition to
COVAX, Operation Warp Speed (OWS)-the U.S. administration's national program
for the countermeasure associated with coronavirus detection, therapeutics, and
vaccine development-holds the key objective of launching a safe vaccine by the
end of January 2021. This is a public-private partnership between private
firms, federal agencies, the Departments of Defense, Energy, & Veteran
Affairs, and major operating components of Health & Human Services, such as
the Centers for Disease Control and Prevention (CDC), National Institutes of
Health (NIH), and the U.S FDA (Food and Drug Administration), among others.
The market is also
marked by several controversies, such as the United States’ decision of
non-participation in the COVAX initiative. However, at the start of September
2020, the European Commission confirmed its interest in participation in the
COVAX initiative and also announced about USD 478 million contributions.
Countries such as South Africa are urging the nations for active participation
in the initiative. India’s Serum Institute is expected to receive USD 150
million at-risk funds for the rapid manufacturing of vaccines co-developed by
the University of Oxford, drug-maker AstraZeneca, and a vaccine development
company Novavax. This collaboration also involves GAVI, the vaccine alliance
that leads to COVAX. GAVI will be providing Pune-based manufacturers with
upfront capitals through the foundation’s Strategic Investment Funds.
Since the COVID-19
vaccine is not a commercial market, funding remains a major concern in the
pandemic situation. Small companies can benefit through guidance received from
the Biomedical Advanced Research and Development Authority (BARDA). BARDA can
aid in the facilitation of dialogue between regulatory agencies and guide trial
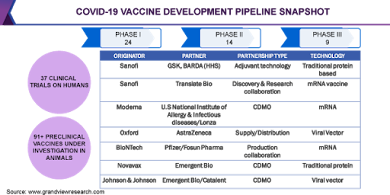
design and expectations of regulatory agencies. Sanofi has partnered with BARDA
and GSK for its traditional protein-based vaccine based on the baculovirus
expression system.
Case Fatality Ratio
(CFR) and Quality Adjusted Life Year (QALY) are to be taken into consideration
from the payors’ and manufacturers’ perspective. Pricing for the vaccines is
usually set differently form the drug-pricing equation. The pricing also
depends on the presence of COVID-19, which has encouraged immunologists to
develop exciting platforms to increase focus on infections, for a change. The
industry has been motivated toward investments and research about cancer
therapies and, thus, the diversified global biopharmaceutical business needs
favorable investments in the vaccinology domain.
Geographically,
leading developers of the COVID-19 vaccine are majorly located across 19
countries; dominated by countries in North America, followed by China, and
European and Asian countries (excluding China) in the league. Private entities
and academic institutes are leading profiles in the vaccine development across
the globe. Researchers are incorporating novel technologies that never have
been implemented as a licensed vaccine previously.
Press Release @ https://www.grandviewresearch.com/press-release/global-covid-19-clinical-trials-market
Viral vector vaccines, virus vaccines, nucleic
acid-based vaccines, and protein-based vaccines are being tested in the clinical
trials. Virus vaccines include weakened and inactivated viruses. Protein-based
vaccines are dominating in terms of the number of clinical trials. Most of the
developers are focusing on receptor binding domain or spike protein.
Determination of the initial clinical efficacy of the vaccine under development
is difficult. Identification of a vaccine platform that provides durable
protection against SARS-CoV-2 along with optimal immunogenicity needs to be
found and manufactured on a large scale. However, other challenges that would
arise post-approval include quality assurance, reliable distribution, standing
high on cold-chain requirements, and other bottlenecks such as medical glass
shortage that can affect timely vaccine packaging and delivery. Thus, companies
are collaborating to scale-up their production facilities. For instance, the
collaboration of Moderna with Lonza, BioNTech with Pfizer, and AstraZeneca with
Serum Institute of India and Pfizer.
Furthermore, the
risk of virus mutation is being evaluated by several researchers. Most of the
researchers are focused on monitoring and evaluating Spike (S) protein, as this
protein mediates infection in the human cells and is the target of most
vaccine/ antibody strategies. Theories also suggest that a vast majority of
mutations are less advantageous to the virus and generally reduce its
transmissibility and pathogenesis.
Companies are working in collaborations to achieve
the best possible results and introduce a vaccine as early as possible. GSK has
adopted a strategic approach for supporting the rapid development of vaccines.
Major companies include, but are not limited to, Pfizer, Moderna, BioNtech,
Sanofi, Zydus Cadila, CureVac, Imperial College London, Takara Bio, CanSino
Biologics, Beth Israel Deaconess Medical Center, ReiThera, Novartis, Anhui
Zhifei Longcom, Novavax, and the University of Queensland. Sanofi is working
via two approaches targeting different pandemic capacities. Moderna is
developing vaccines that make use of messenger-RNAs to deliver viral proteins
in the body. The company is also funded with USD 1 billion by the government.
About Us:
Grand
View Research, Inc. is a U.S. based market research and consulting company,
registered in the State of California and headquartered in San Francisco. The
company provides syndicated research reports, customized research reports, and
consulting services. To help clients make informed business decisions, the
company offers market intelligence studies ensuring relevant and fact-based
research across a range of industries including technology, chemicals, materials,
healthcare and energy.
Contact:
Sherry James
Corporate Sales Specialist, USA
Grand View Research, Inc
Phone: 1-415- 349-0058
Toll Free: 1-888- 202-9519
Email: sales@grandviewresearch.com
For More Information: https://www.grandviewresearch.com
No comments:
Post a Comment